Additional Resources
- Access the interactive tool here
- Read the Guideline At-a-Glance here
- Visit the guideline hub here
Today, I review excerpts from Section 3 Evaluation, Diagnosis, and Risk Stratification from ACC 2023 Chronic Choronary Disease Guideline.
2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines [PubMed Abstract] [Full-Text HTML] [Full-Text PDF]. Circulation. 2023 Aug 29;148(9):e9-e119. doi: 10.1161/CIR.0000000000001168. Epub 2023 Jul 20.
All that follows is from the above resource.
3 Evaluation, Diagnosis, and Risk Stratification
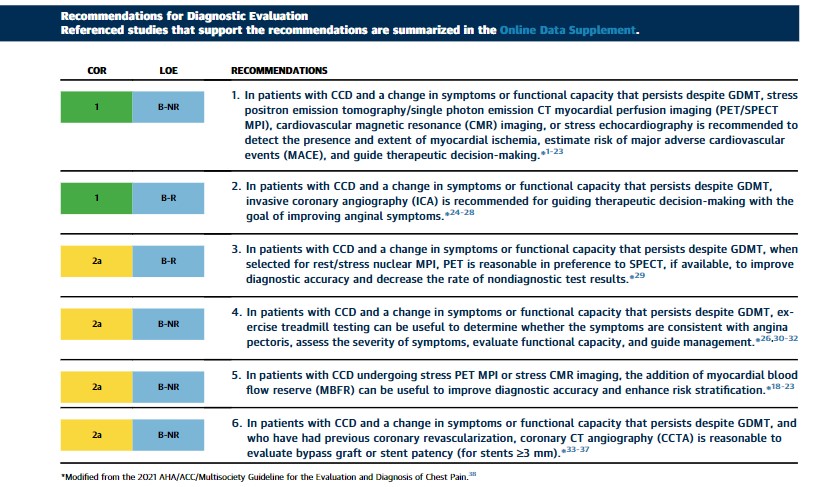
3.1 Diagnostic Evaluation
Synopsis
In patients with CCD, if there is an opportunity to do so, clinicians should first intensify GDMT and defer testing. In patients with CCD, assessing the severity of ischemia may be useful to guide clinical decision-making regarding the use of ICA and for intensification of preventive and anti-ischemic therapy. Imaging should be considered in those with new-onset or persistent stable chest pain. In patients with CCD and frequent angina or severe stress-induced ischemia, referral to ICA or CCTA is an option.26 For additional recommendations about known obstructive and nonobstructive CAD, suspected ischemia, ischemia with nonobstructive coronary arteries (INOCA), role of invasive testing, and revascularization, refer to the 2021 AHA/ACC chest pain guideline,38 the 2021 ACC/AHA/SCAI revascularization guideline,39 as well as Section 6.1.2 (“Ischemia With Nonobstructive Coronary Arteries”) of this guideline. Additionally, cost–value considerations for diagnostic testing contained within the 2021 AHA/ACC chest pain guideline, Section 5.3, should be considered.38
Recommendation-Specific Supportive Text
1. Observational studies reveal that patients with moderate to severe ischemia on PET and SPECT MPI have an improved outcome with early coronary revascularization.7,21,40-43 Clinical trials of CMR imaging that included subgroups of patients with obstructive CAD, showed comparable diagnostic accuracy to stress SPECT MPI.10,11 Several large, multicenter registries revealed that stress CMR imaging effectively risk stratifies patients with known CAD.14-17 In a multicenter registry of 2,496 patients with a history of CAD, an abnormal stress CMR image was associated with a nearly 2-fold increased mortality hazard.14 Registry data also reported that patients with chest pain syndrome with ischemia by MPI and scarring by late gadolinium enhancement had a relative hazard of 1.5 to 2.1 for cardiovascular death or nonfatal MI.17 Prognosis worsens for patients by the extent and severity of inducible wall motion abnormalities on stress echocardiography.44,45 Recent randomized trial evidence supports the role of stress echocardiography to guide clinical decision-making. From the ORBITA (Objective Randomized Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina) trial, a secondary outcome was a greater reduction in the stress echocardiographic wall motion score among patients with single-vessel CAD treated with percutaneous coronary intervention (PCI) compared with placebo (P<0.0001).46 Patients with PCI and who have a wall motion score ≥1 were more often angina-free compared with those in the placebo arm.
2. Randomized trials of patients with CCD reveal a pattern that ischemia-guided PCI results in an improvement in angina when compared with medical therapy alone.24-26,47,48 In the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical & Invasive Approaches) trial, 5,179 patients with stable CAD and site-determined moderate-severe ischemia on stress testing (patients with ≥50% left main stenosis on CCTA, left ventricular ejection fraction [LVEF] <35%, and unacceptable angina on medical therapy were excluded) were randomized to invasive versus conservative care strategies.26 No difference in the composite primary MACE (cardiovascular death, MI, hospitalization for unstable angina, HF, or resuscitated cardiac arrest) endpoint was observed at ∼3.3 years of follow-up. Angina symptoms improved in both the conservative and invasive treatment arms, although improvements were larger in the invasive arm, particularly with more frequent angina at baseline.48 Therefore, in patients with CCD with known anatomy and ongoing angina despite GDMT, early invasive angiography and revascularization should be considered to improve symptoms. Notably, secondary analyses of RCTs have reported no differences in major adverse cardiovascular outcomes in medical versus invasive medical treatment strategies in patient with CCD49 when stratified by ischemia severity on noninvasive testing.
3. The improved diagnostic accuracy of PET MPI is helpful in patients with known CAD. In a randomized trial of 322 symptomatic patients with known CAD, the presence of low- and high-risk stress PET findings was associated with lower and higher rates of ICA when compared with SPECT MPI (P=0.001).29
4. Observational studies of patients with CAD and stable chest pain have shown that exercise treadmill testing can be useful by evaluating the relation of symptoms to graded stress testing, thereby helping to confirm the diagnosis of angina pectoris; assessing symptom severity; and selecting appropriate management (eg, medical therapy, revascularization, cardiac rehabilitation [CR]).26,30-32
5. Reduced MBFR reflects abnormalities of flow within the epicardial coronary arteries, microvasculature, or both, and independently predicts risk of major CAD events. Measurement of MBFR can be effectively accomplished using PET18,50,51 or CMR.15 Normal MBFR may be helpful in excluding high-risk anatomy, although global reduced levels (<2) may provide a better estimate of disease extent and severity. Nonobstructive CAD with reduced MBFR is more frequently observed in women.50
6. CCTA is accurate for the assessment of native vessel CAD and bypass graft patency with high accuracy (∼96%) and concordance (82% to >93%) to ICA. It may also be useful to assess patency of proximal large stents (≥3 mm) if such information is known at the time of presentation.33-37 Other modalities may be considered in patients with CCD with smaller or more distal stents. Several controlled clinical trials have evaluated the concordance of fractional flow reserve (FFR)-CT with invasive FFR.52-55 Diagnostic sensitivity of FFR-CT compared with invasive FFR is high.19,53
3.2 Risk Stratification and Relationship to Treatment Selection
Recommendations for Risk Stratification and Relationship to Treatment Selection
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
COR LOE Recommendations Risk Stratification and Prognosis 1 B-NR
1. In patients with CCD, it is recommended that risk stratification incorporate all available information, including noninvasive, invasive, or both cardiovascular diagnostic testing results or use validated risk scores to classify patients as low (<1%), intermediate (1%-3%), or high (>3%) yearly risk for cardiovascular death or nonfatal MI.1-4
Relationship to Treatment 1 A
2. In patients with CCD, optimization of GDMT is recommended to reduce MACE.∗ 5-7
1 A
3. In patients with CCD with newly reduced LV systolic function, clinical heart failure, or both, ICA is recommended to assess coronary anatomy and guide potential revascularization.8,9
3: No benefit A
4. In patients with CCD, ICA for risk stratification is not routinely recommended in patients without LV systolic dysfunction, heart failure, stable chest pain refractory to GDMT, and/or noninvasive testing suggestive of significant (>50%) left main disease.5-7,10,11
*c-index: Pitfalls of the concordance index for survival outcomes. [PubMed Abstract]. Nicholas Hartman 1, Sehee Kim 2, Kevin He 1, John D Kalbfleisch 1
Abstract
Prognostic models are useful tools for assessing a patient’s risk of experiencing adverse health events. In practice, these models must be validated before implementation to ensure that they are clinically useful. The concordance index (C-Index) is a popular statistic that is used for model validation, and it is often applied to models with binary or survival outcome variables. In this paper, we summarize existing criticism of the C-Index and show that many limitations are accentuated when applied to survival outcomes, and to continuous outcomes more generally. We present several examples that show the challenges in achieving high concordance with survival outcomes, and we argue that the C-Index is often not clinically meaningful in this setting. We derive a relationship between the concordance probability and the coefficient of determination under an ordinary least squares model with normally distributed predictors, which highlights the limitations of the C-Index for continuous outcomes. Finally, we recommend existing alternatives that more closely align with common uses of survival models.
Keywords: concordance index; prognostic modeling; risk discrimination; survival analysis.
© 2023 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Resuming excerpts from Section 3
Recommendation-Specific Supportive Test (continued)
2. The 2021 AHA/ACC chest pain guideline recommends the optimization of anti-ischemic and preventive therapies with the goal to reduce the patient’s angina burden and improve clinical outcomes.12 Three major RCTs including COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), ISCHEMIA, and BARI-2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) have shown that there is no reduction in MACE with routine cardiovascular revascularization.5-7 The COURAGE trial, which included patients with stabilized Canadian Cardiovascular Society class IV angina and at least a 70% stenosis in at least 1 coronary artery with evidence of ischemia, reported no difference in all-cause death or nonfatal MI between revascularization with PCI and optimal medical therapy. The BARI-2D trial randomized patients with type 2 diabetes and CCD (≥70% stenosis of a major coronary artery and angina or ≥50% stenosis of a major coronary artery with a positive stress test) to revascularization or medical therapy and reported no difference in survival.
3. The STICH (Surgical Treatment for Ischemic Heart Failure) trial randomized 1,212 patients with an ejection fraction ≤35% with coronary disease amenable to coronary artery bypass grafting (CABG) to either medical therapy alone or medical therapy and CABG. After a median follow-up of 56 months, no significant difference was observed in the primary outcomes of all-cause death (41% versus 36%; P=0.12), but cardiovascular death (33% versus 28%; P=0.05) and all-cause death or cardiovascular hospitalization (68% versus 58%; P<0.001) were lower in the CABG arm.9 In a secondary analysis of the ISCHEMIA trial, 398 participants had HF or an LVEF <45%. Both the 4-year primary composite endpoint (17.2% versus 29.3%; event rate difference, −12.1% [95% CI, −22.6 to −1.6]) and cardiovascular death or MI (14.6% versus 25.9%; event rate difference −11.4% [95% CI, −21.4 to −1.4]) were lower in the invasive treatment arm.8 The REVIVED-BCIS2 trial randomized 700 patients with and LVEF ≤35% with CCD amenable to PCI to either medical therapy or PCI plus medical therapy and reported no difference all-cause death or health failure hospitalization (38.0% versus 37.2%).51 In addition to revascularization, ICA can also help diagnose the cause of HF and help direct medical therapies (eg, lipid lowering). We acknowledge the data are less robust for patient with HF with preserved ejection fraction.9 Noninvasive modalities may be appropriate to evaluate for coronary ischemia in some circumstances. Alternatively, CCTA may be considered as an initial diagnostic strategy in selected patients with suspected nonischemic cardiomyopathy.52
4. Three multicenter trials (COURAGE, BARI 2D, ISCHEMIA) showed no improvement in clinical endpoints in patients with CCD randomized to routine revascularization plus GMDT or initial GDMT; although 21% to 42% of patients randomized to GDMT eventually underwent revascularization.5-7 In a secondary analysis of the ISCHEMIA trial, although ischemia severity on noninvasive testing was associated with all-cause death, no treatment interaction was observed when participants were stratified by mild, moderate, or severe ischemia.10 Similarly, in a secondary analysis of the COURAGE trial limited to the 60% of patients with stress perfusion imaging and coronary angiography information available, there was no interaction between therapeutic strategy and either severity of ischemia or coronary anatomy.11